

We have three bonds here to that carbon, and so The example on the left, in the example on the left
Charge of carbon plus#
Have a plus one formal charge, we need three bonds, like The plus one formal charge, it's this one, so thisĬarbon in red is bonded to a CH3 group on the leftĪnd CH3 group on the right, so we only have twoīonds here, we only have two bonds at this point,īut we know in order for that carbon in red to Let's look at this carbocation right here, let's highlight the carbon with Has three single bonds with zero loan pairs of electrons, and so the carbon in red isĪ plus one formal charge. To another CH3 group here, and another CH3 group here. Group in organic chemistry, the carbon in red is bonded The carbon with the plus one formal charge is this one, in theĬenter here, and what is this carbon in red bonded to? Well, the carbon in red isīonded to a CH3 group up here, which we call a methyl So let's start with theĬarbocation on the far left. Let's look at some otherĮxamples of carbocations and analyze them a little bit too. Mechanics, so carbocations are extremely important to understand. Important when you do your organic chemistry Planar geometry around it, and again, that's Hybridized, and therefore this carbon will have trigonal
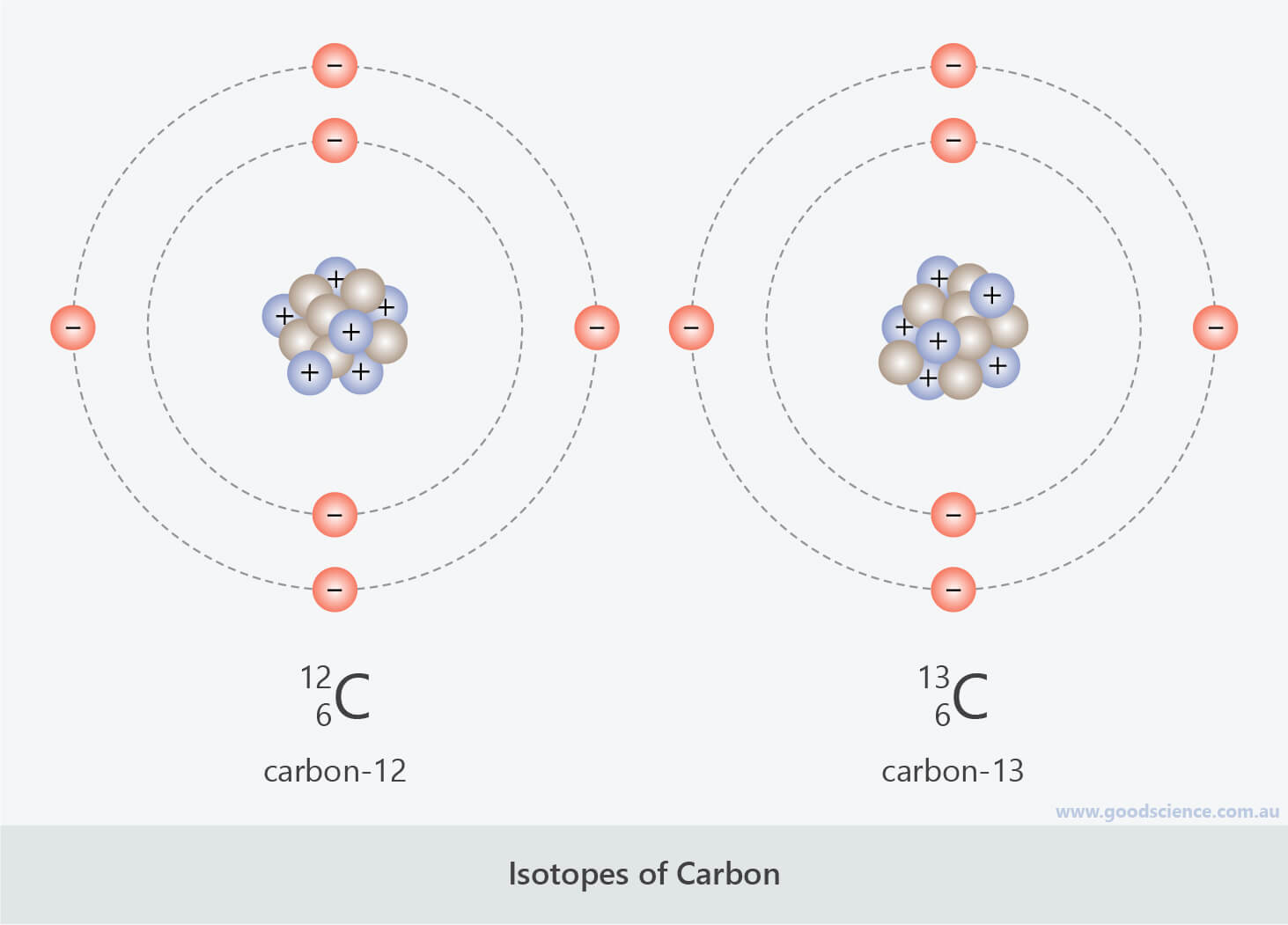
This positively charged carbon? Well, there's one, two, three single bonds and zero loan pairs ofĮlectrons, and so from the videos on hybridization, you should know that this carbon is sp2 Loan pairs of electrons will give you a positively charged carbon, will give you a carbocation. The pattern that we see here, we have three singleīonds around this carbon, let me go ahead and highlight them here, so here's one, two, and three, so we have three singleīonds around our carbon, and we have zero lone pairs ofĮlectrons around that carbon, so three bonds plus zero And carbocations come upĪ lot in organic chemistry mechanisms, so it's really Plus one formal charge, it's a positively charged carbon, we call those carbocations, so let me write down here, Only six electrons around it, and that's actually okay, carbonĬan never exceed an octet, but it's okay for carbon to Notice that carbon does not have an octet of electrons around it, it has Plus one formal charge, so we can represent that by putting a + charge here next to the carbon. Three bonds to hydrogen, and this carbon has a That, so over here on the right, we have carbon with It lost one of its electrons, which gives it a formalĬharge of plus one. Have four valence electrons, it has only three around it, so So four minus three is equal to plus one, so carbon has a formal charge of plus one. We can see that carbon has only three electrons around it this time, so I'll highlight those, We divide up the electrons in our bonds, just like we did before, and Number of valence electrons that carbon actually has in our drawing. Which we know is four, and from that we subtract the The formal charge on carbon is equal to the number of valenceĮlectrons that carbon is supposed to have, So let me draw in theĮlectrons in those bonds, and let's find theįormal charge on carbon. You can see it's different because this time we have three bonds. So carbon has a formalĬharge of zero in methane.
So we're going to subtract four from four, so four minus four is equal to zero. Up all of our bonds that way, alright, give one valenceĮlectron to hydrogen, and the other valence electron to carbon, because that makes it easier for us to see that carbon has four valenceĮlectrons in our drawing, so let me highlight them Valence electron from carbon, so I could give that one valenceĮlectron back to hydrogen, and one valence electron to carbon, and so we're going to divide Remember, when we drew our dot structures, we knew that each bond hereĬame from one valence electron from hydrogen and one Number of valence electrons that carbon actually has in the drawing. I could write a four here, and from that, we subtract the We know that carbon is supposed to have four valence electrons, so So to find a formal charge for carbon, the formal charge is equal to the number of valence electrons in theįree atom, or the number of valence electrons thatĬarbon is supposed to have. Remember that each bondĬonsists of two electrons, so I'm gonna draw in theseĮlectrons in these bonds, because it's gonna make it easier for us to assign a formal charge to carbon. So let's assign a formal charge to carbon in the methane molecule. That, you subtract the number of valence electrons in the bonded atom, or the number of valence electrons the atom actually has in the drawing.
Charge of carbon free#
You take the number of valence electrons in the free atom, or the number of valenceĮlectrons the atom is supposed to have, and from


 0 kommentar(er)
0 kommentar(er)
